¡Puaj! 10+ Raras razones para el Ionic Compound Naming With Transition Metals Worksheet: Naming ionic compounds with transition metals.
Ionic Compound Naming With Transition Metals Worksheet | Metals combine with nonmetals to give ionic compounds. Model 3 — ionic compound names (metals that form multiple ions) cu o copper(l) oxide cuo copper(ll) oxide snf tin (il) fluoride snf4 tin(1v) fluoride Do not use prefixes to indicate how many of each element is present; In this part of the worksheet, you will learn to easily classify compounds into one of the two categories. Learn about the formation of covalent compounds, the properties and naming of covalent.
For example, iron (fe) can form the iron (ii) ion and also the iron (iii) ion, denoted fe 2+ and fe 3+, respectively. A roman numeral (i, ii, iii, iv, v, …) must be used in the cation and ionic compound naming system to distinguish between the charges. Metals combine with nonmetals to give ionic compounds. In this part of the worksheet, you will learn to easily classify compounds into one of the two categories. Learn about the formation of covalent compounds, the properties and naming of covalent.
Learn about the formation of covalent compounds, the properties and naming of covalent. H lewis dot diagrams lewis dot diagrams are a way to indicate the number of valence electrons around an atom. Some transition metals have multiple possible cation charges. For example, iron (fe) can form the iron (ii) ion and also the iron (iii) ion, denoted fe 2+ and fe 3+, respectively. We'll see how you have to balance the charges of the two ions so they cancel each other out. Hydrogen ionic bonding occurs when a metal transfers one or more electrons to a nonmetal in an effort … The key to naming ionic compounds with transition metals is to … When naming compounds, remember the … Naming ionic compounds with transition metals. Don't use "mono" for first name, but always for second name. Here's how to write formulas for binary ionic compounds. Do not use prefixes to indicate how many of each element is present; Metals combine with nonmetals to give ionic compounds.
The classification of a compound depends on the type of chemical bond between the elements in the compound. Don't use "mono" for first name, but always for second name. 26.10.2017 · ionic compound demo observation. For example, iron (fe) can form the iron (ii) ion and also the iron (iii) ion, denoted fe 2+ and fe 3+, respectively. We'll see how you have to balance the charges of the two ions so they cancel each other out.
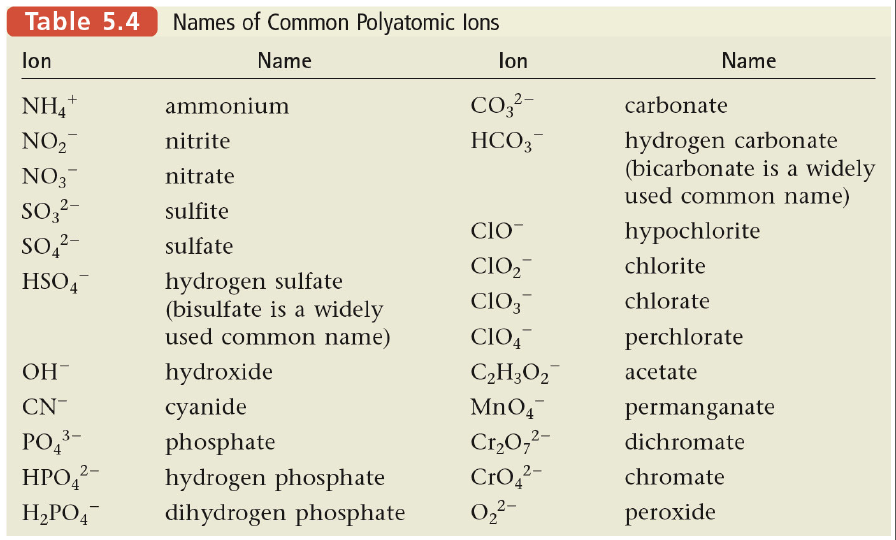
When naming compounds, remember the … Some transition metals have multiple possible cation charges. For example, iron (fe) can form the iron (ii) ion and also the iron (iii) ion, denoted fe 2+ and fe 3+, respectively. This can make naming confusing. Do not use prefixes to indicate how many of each element is present; A roman numeral (i, ii, iii, iv, v, …) must be used in the cation and ionic compound naming system to distinguish between the charges. Learn about the formation of covalent compounds, the properties and naming of covalent. Covalent compounds ionic compounds ; Transition metals make naming and formula writing a bit more challenging. H lewis dot diagrams lewis dot diagrams are a way to indicate the number of valence electrons around an atom. Hydrogen ionic bonding occurs when a metal transfers one or more electrons to a nonmetal in an effort … Model 3 — ionic compound names (metals that form multiple ions) cu o copper(l) oxide cuo copper(ll) oxide snf tin (il) fluoride snf4 tin(1v) fluoride Don't use "mono" for first name, but always for second name.
Naming compounds what's it made of? We'll see how you have to balance the charges of the two ions so they cancel each other out. Don't use "mono" for first name, but always for second name. In this part of the worksheet, you will learn to easily classify compounds into one of the two categories. 26.10.2017 · ionic compound demo observation.

Here's how to write formulas for binary ionic compounds. Covalent compounds ionic compounds ; When naming compounds, remember the … The classification of a compound depends on the type of chemical bond between the elements in the compound. Naming compounds what's it made of? Naming ionic compounds with transition metals. The key to naming ionic compounds with transition metals is to … Hydrogen ionic bonding occurs when a metal transfers one or more electrons to a nonmetal in an effort … Some transition metals have multiple possible cation charges. Do not use prefixes to indicate how many of each element is present; Transition metals make naming and formula writing a bit more challenging. For example, iron (fe) can form the iron (ii) ion and also the iron (iii) ion, denoted fe 2+ and fe 3+, respectively. In this part of the worksheet, you will learn to easily classify compounds into one of the two categories.
Ionic Compound Naming With Transition Metals Worksheet! Model 3 — ionic compound names (metals that form multiple ions) cu o copper(l) oxide cuo copper(ll) oxide snf tin (il) fluoride snf4 tin(1v) fluoride
0 Response to "¡Puaj! 10+ Raras razones para el Ionic Compound Naming With Transition Metals Worksheet: Naming ionic compounds with transition metals."
Post a Comment